- 59 new drugs approved by FDA in 2018, an all-time high
- Government shutdown likely to weigh on approvals of drugs for life-threatening conditions in 2019
The U.S. Food and Drug Administration (“FDA”) approved a record-setting 59 new molecular entities in 2018.
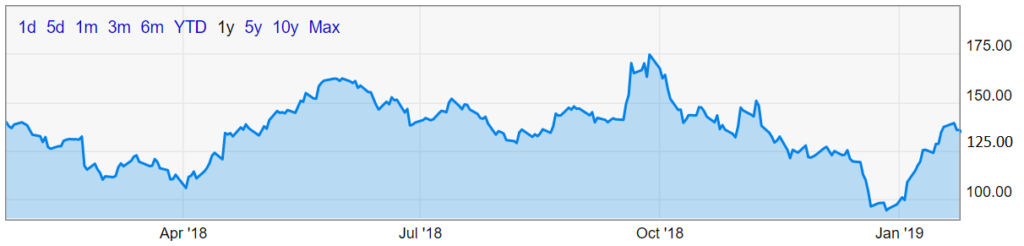
Notable among the approvals was the first marijuana-extract Epidiolex, used to treat a rare form of epilepsy. Epidiolex was developed by GW Pharmaceuticals, plc (NASDAQ: GWPH), whose shares have been extremely volatile in the past twelve months, trading as high as $179.65 before dropping as low as $90.14 this past December.
A list of drugs approved by the FDA in 2018, their intended target or ‘indication’ and how they work or their ‘mode of action’ is listed in the table below – hat tip to Forbes contributor Bernard Munos for putting this together.

FDA Drug Approvals Peak At All-Time High in 2018
Article By: Fatimah Aminu
Fatimah is an experienced editor at various financial and consumer publishing houses. She obtained a master’s degree in Publishing from NYU, where she earned a bachelor of fine arts degree. She is currently earning a second masters degree at CUNY online in Psychology. Fatimah covers healthcare, cannabis and technology.
